Most fluids offer some resistance to motion, and we call this resistance “viscosity.” Viscosity arises when there is relative motion between layers of the fluid. More precisely, it measures resistance to flow arising due to the internal friction between the fluid layers as they slip past one another when fluid flows. Viscosity can also be thought of as a measure of a fluid’s thickness or its resistance to objects passing through it.

Viscosity
A fluid with large viscosity resists motion because its strong intermolecular forces give it a lot of internal friction, resisting the movement of layers past one another. On the contrary, a fluid with low viscosity flows easily because its molecular makeup results in very little friction when it is in motion. Gases also exhibit viscosity, but it is harder to notice in ordinary circumstances.
In this article, Pritish Kumar Halder gives a brief description of Viscosity.
Viscosity Definition
The definition of viscosity is as follows:
Viscosity is a measure of a fluid’s resistance to flow.
The SI unit of viscosity is poiseiulle (PI). Its other units are newton-second per square metre (N s m-2) or pascal-second (Pa s.) The dimensional formula of viscosity is [ML-1T-1].

Viscosity unit
The viscosity of liquids decreases rapidly with an increase in temperature, and the viscosity of gases increases with an increase in temperature. Thus, upon heating, liquids flow more easily, whereas gases flow more slowly. Also, viscosity does not change as the amount of matter changes, therefore it is an intensive property.
Viscosity Formula
Viscosity is measured in terms of a ratio of shearing stress to the velocity gradient in a fluid. If a sphere is dropped into a fluid, the viscosity can be determined using the following formula:
η=(2ga^2 (∆ρ))/9v)
Where ∆ρ is the density difference between fluid and sphere tested, a is the radius of the sphere, g is the acceleration due to gravity and v is the velocity of the sphere.
Viscosity Types
Viscosity is the measure of fluid’s friction to its flow. There are two ways to measure the fluid’s viscosity as follows:
- Dynamic Viscosity (Absolute Viscosity)
- Kinematic Viscosity

Viscosity Types
One way is to measure the fluid’s resistance to flow when an external force is applied. This is known as Dynamic Viscosity. And the other way is to measure the resistive flow of a fluid under the weight of gravity. We call this measure of fluid viscosity kinematic viscosity.
Many are confused between the two viscosity measures and consider them to be one and the same. In reality, they have significant differences between them. For a few applications, kinematic viscosity is more useful than absolute or dynamic viscosity.
Newtonian and Non-Newtonian Fluids

Newtonian and Non-Newtonian Fluids
Temperature and pressure affect the viscosity of a fluid. The viscosity of liquids increases rapidly with an increase in temperature. On increasing pressure viscosity of liquid molecules increases due to the increase in the resistance to the flow of liquid.
If the viscosity does not change with pressure, we describe something as being a Newtonian fluid. And, if the viscosity does change as stress or temperature changes, we describe something as being a non-Newtonian fluid. Water is an example of Newtonian fluid and toothpaste is an example of Non-Newtonian fluid.
Viscosity Measurement
The elementary way of measuring viscosity is to allow a sphere, such as a metal ball, to drop through a fluid and time the fall of the metal ball. The slower the sphere falls, the greater the viscosity. But, a more accurate measure of viscosity is given by the viscometer.
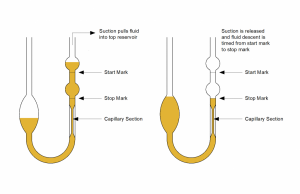
U-Tube Viscometer

U- Tube viscometer
U-tube viscometers are also known as glass capillary viscometers or Ostwald viscometers.
A viscometer consists of two reservoir bulbs and a capillary tube. In one arm of the U is the capillary, a vertical section of a precise narrow bore. Above, which is a bulb, and with it is another bulb lower down on the other arm, as shown in the image.
In use, the upper bulb draws the liquid by suction, and then the liquid is made to flow down through the capillary into the lower bulb. Two marks (one above and one below the upper bulb) indicate a known volume. The time taken for the liquid to pass between these marks is proportional to the kinematic viscosity.
Most commercial units are provided with a conversion factor. The time taken by the test liquid to flow between two points is measured. By multiplying the time measured by the factor of the viscometer, the kinematic viscosity is obtained.
Factors affecting viscosity –
Pressure has an effect on both, the viscosity of liquid as well as gases.
- On increasing pressure viscosity of liquid molecules increases due to the increase in the resistance to the flow of liquid.
- On increasing pressure, the viscosity of gas molecules decreases due to the increase in glow of molecules. Under most conditions, viscosity is independent of pressure. Viscosity has a pressure dependence for gases when the ideal gas model breaks down (e.g. at low temperatures and/or high pressures) and for liquids at very high pressure.
- Under most circumstances, viscosity is sovereign of pressure. Viscosity has a pressure dependence for gases when the ideal gas model breaks down (e.g. at low temperatures and/or high pressures) and for liquids at very high pressure.
- Viscosity is usually independent of pressure, but liquids under extreme pressure often experience an increase in viscosity. Since liquids are usually incompressible, an augment in pressure doesn’t actually bring the molecules appreciably closer together.
- The viscosity of gases increases as temperature increases and is around comparative to the square root of temperature. This is due to the augment in the incidence of intermolecular collisions at higher temperatures.
There is no effect of pressure on the coefficient of viscosity of gases. For most circumstances near the conditions we live in, pressure doesn’t have much effect on viscosity.